Project
Summary and objectives
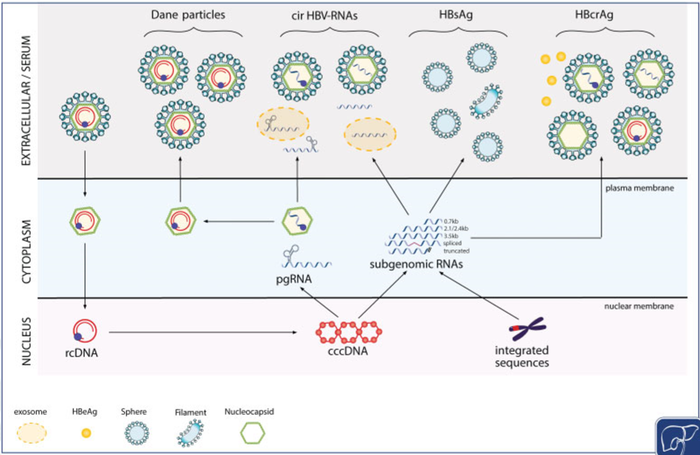
The 257 million chronic HBV infections remain a major health burden worldwide. Only 1.7 million patients are currently treated for their infection. However, this pathology can lead to chronic liver inflammation, cirrhosis or even to hepatocellular carcinoma, the second leading cause of cancer deaths in the world. Current treatments for chronic hepatitis B include pegylated interferon alpha2a and nucleos(t)ide analogues (NUC). Only a limited number of treated patients achieve the loss of hepatits B surface antigen (HBsAg) that defines a functional cure of HBV infection allowing treatment cessation and is associated with a decreased risk of liver complications. A key challenge for drug development is to target the pool of cccDNA, the nuclear replicative intermediate that acts as template for the transcription of all viral mRNAs. Several new drugs have reached the preclinical and/or clinical evaluation with the aim of silencing cccDNA and/or reducing the size of the cccDNA pool to achieve a functional cure with finite treatment duration. The working hypothesis is that cirB-RNAs reflects the transcriptional activity of the cccDNA in the liver and would be the best biomarker for the reduction of the cccDNA pool and it functional inactivation. The goals of the CirB-RNA project are first the validation of the circulating hepatitis B RNAs as an innovative, clinically relevant and non invasive biomarker to predict a functional cure ; and second the development and validation of the related diagnostic test.
In parallel with the CirB-RNA project, the IP-Cure-B project (https://ipcureb.eu/) started in January 2020 for a period of 5 years.
The objective of the EU-funded IP-cure-B project is to develop novel curative concepts for chronic hepatitis B (CHB). Specifically, this project aims to (1) improve the cure rate of CHB by boosting innate immunity with immune modulators and stimulating adaptive immune responses with a novel therapeutic vaccine, (2) characterize immune and viral biomarker signatures for patient stratification and treatment response monitoring, (3) integrate biological and clinical data to model the best combination treatment for future trials, and (4) model the effectiveness of novel curative therapies with respect to disease spectrum, patient heterogeneity, and constraints of National Health Systems.
This project is funded by the European Union’s Horizon 2020 research and innovation programme under grant agreement n°847939 (https://ec.europa.eu/) under the topic (https://cordis.europa.eu/programme/id/H2020_SC1-BHC-14-2019)
The project is coordinated by the Université Claude Bernard de Lyon (Discover Lyon 1 - Université Claude Bernard Lyon 1 (univ-lyon1.fr)) and by Pr Zoulim.
Several partners are involved in the project (https://ipcureb.eu/principal-investigators/).
For more information :
- Global Hepatitis Report 2017—World Health Organization, April 2017: http://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/
- WHO: http://www.who.int/hepatitis/en/
- Global Hepatitis Report 2017—World Health Organization, April 2017
- WHO Global Hepatitis Report, 2017
- Global Health Sector Strategy on Viral Hepatitis 2016−2021. WHO. July 2016.
- United Nations General Assembly Resolution A/RES/70/1—Transforming our world: the 2030 Agenda for Sustainable Development, see http://www.un.org/ga/search/view_doc.asp?symbol=A/RES/70/1&Lang=E (accessed 1 June 2017)
- Global Health Sector Strategy on Viral Hepatitis 2016-2021. WHO. July 2016
Benchmarking of circulating HBV RNAs as a marker for functional healing of chronic hepatitis B